Chemical Properties of Phenols
Chemical Properties of Phenols: Overview
This Topic covers sub-topics such as Schotten-Baumann Reaction, Nitration of Phenols, Chemical Properties of Phenols, Kolbe's Reaction of Phenol, Reduction of Phenols, Reaction of Phenol with Zinc Dust, Phthalein Reaction and, Nitrosation of Phenol
Important Questions on Chemical Properties of Phenols
When phenol is heated with phthalic anhydride in presence of conc. , an important acid-base indicator is formed.
When phenol is heated with phthalic anhydride in presence of conc. , an important acid-base indicator is formed. Identify its structure.
On heating phenol with phthalic anhydride in presence of conc. , a product X is obtained. X is
Complete the following reaction:
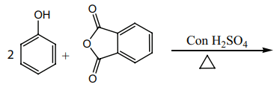
Explain the phthalein reaction of phenol.
The hydrogenation of phenol in the presence of nickel and heat gives
The catalytic hydrogenation of phenol gives cyclohexanol.
The catalytic hydrogenation of phenol gives _____.
Describe catalytic hydrogenation of phenol.
Phenol can be readily nitrosated at high temperature with .
Which of the following reagent will convert phenol to p-nitrosophenol?
Complete the following reaction:
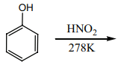
How will you convert phenol to p-nitrosophenol.
Phenol when treated with in presence of anhydrous at and high pressure gives aniline.
Which of the following reagent will convert phenol to aniline?
Complete the following reaction:
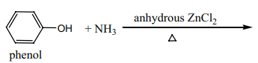
Convert phenol to aniline.
What is etherification of phenol?
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Phenols give and nitrophenol on nitration with conc. and mixture.
Reason : group in phenol is directing.
Assertion : Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason : In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
